Sanner's end-to-end CDMO services –
Precision Engineered. Seamlessly Delivered.
Sanner Group provides seamless end-to-end solutions for the design, development, and contract manufacturing of drug delivery systems, combination products, medical devices, digital health products, and diagnostics – the full spectrum of everything from simple, single-use consumables to complex devices.
As a CDMO for medical devices we offer substantial molding, assembly and kitting capacity paired with agility and scalability to bring your products to market efficiently and effectively. Our state-of-the art manufacturing facilities in Europe, North America and Asia make Sanner a strong partner of choice whether for small production runs or high-volume manufacturing.
Don’t miss our presentations at PODD and CPHI!
- PODD
- Monday, October 27, 2025 – 05:30 PM - 05:45 PM
A Novel Approach to Modelling Injector Performance and Break-Loose Extrusion Force Data
Presenter: Tom Oakley, Springboard, co-presenting with Deep Bhattacharya, Pfizer
- Monday, October 27, 2025 – 05:30 PM - 05:45 PM
- CPHI
- Wednesday, October 29, 2025 – 11:35 AM - 12:05 PM
Rethinking Injector Mechanics – A New Approach to BLEF-Based Modelling
Speakers: Tom Oakley, Springboard, co-presenting with Jay Sayed, Pfizer
- Wednesday, October 29, 2025 – 11:35 AM - 12:05 PM
-
- Wednesday, October 29, 2025 – 12:05 PM - 12:20 PM
Modelling to Market – Enabling Smarter, Faster Injector Development
Speaker: Alex Vasiev, Springboard
- Wednesday, October 29, 2025 – 12:05 PM - 12:20 PM
Meet our experts at the conferences
Feel free to reach out to our sales representatives to arrange a meeting in advance.
Click on the images to send an email.
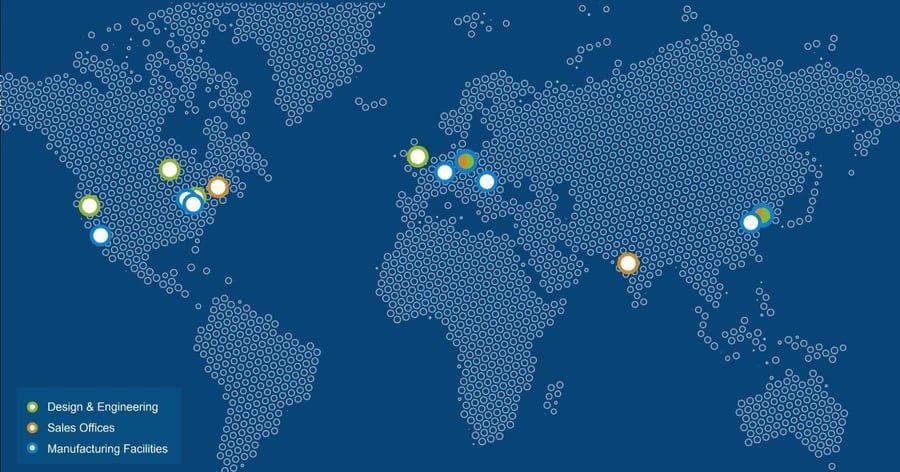
Worldwide production capacity and device development services
Sanner operates a global manufacturing network with a total production space of 28,500 sqm, including 11,000 sqm of cleanroom space (Class 7/8). Our Design Centers of Excellence, Springboard and Gilero, are strategically located in the UK and across the US. Our manufacturing facilities are based in the US, Asia and Europe.
How we can help you
- Capacity for small-batch prototypes and scaling to full production. Our facilities are equipped to handle a wide range of production needs:
- High precision fully electric thermoplastic injection molding capable of handling small shot weights (around 0.1g) up to 64-cavity molds (max. 350T), including 2K molding with thermoplastic elastomers (TPE).
- Manual assembly up to fully automated assembly processes, incorporating mechatronic components for enhanced efficiency and precision.
- End of line packaging for sub-assemblies or finished goods
- Sterilization through trusted external partners, utilizing validated processes to ensure compliance with industry standards.
- Our experience in successfully transferring innovations to production across the EU, US, and China speaks to our ability to bring cutting-edge technologies to global markets. All phases of device development can be supported from our Design and Development Centers of Excellence in both the US and EU.
- Our design transfer processes enable the smoother path to market. We offer end-to-end thinking from development to manufacturing all under one roof to de-risk your project and for a better time to market.
- Our reliability and collaborative working attitude support you in any stage of your project.
Quality control processes and compliance with ISO 13485
Sanner Group facilities are ISO 13485 certified for Design & Development and Manufacturing respectively. Over 250 ISO, AAMI/ANSI standards are used routinely. Our streamlined quality system satisfies the FDA requirements for both the device quality system regulations and the part 210/211 cGMP regulations.
Find out more

Capacity is the new capability.
Meeting market demand.
The demand for weight management drugs, such as GLP-1s, has put immense pressure on manufacturing capacity. The shortage of available manufacturing capacity threatens to delay product launches and limit access for all injectable medicines as more pharmaceutical companies race to launch new drugs and devices for patients.
Find out more in our brochure.

The Rise of the Next-Gen CDMO.
Strategies for Reducing Risk and Time to Market.
Creating and producing advanced drug-device combinations, like autoinjectors and pen injectors, requires a depth and breadth of technical expertise. As more pharmaceutical companies streamline their supply chains and outsource specialised technical tasks, these challenges are increasingly being shifted to their development partners.
More information here.

Does My Medical Device Design Actually Work?
There are few experiences that can surpass the joy of seeing a product you developed reach manufacture. But before that, you face the non-trivial task of proving your current embodiment can actually work and meet its design intent.
Learn more in our PDF.